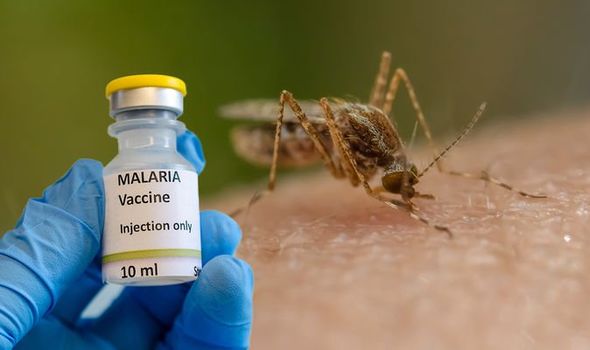
African news. Ghana is the first country to approve a new malaria vaccine that has been described as a “world-changer” by the scientists who developed it.
The Oxford University malaria vaccine has been approved for use in Ghana as the country ramps up efforts to combat malaria to curb child mortality due to the pest.
This is the first time the shot has received regulatory approval anywhere in the world.
The vaccine – called R21 – appears to be hugely effective, in stark contrast to previous ventures in the same field wrote BBC.
“The vaccine has been approved for use in children aged 5-36 months, the age group at highest risk of death from malaria,” the university said in a statement.
Professor Adrian Hill, chief investigator of the R21/Matrix-M vaccine program and director of the university’s Jenner Institute, said it marked the “culmination of 30 years of malaria vaccine research at Oxford with the design and provision of a high efficacy vaccine that can be supplied at adequate scale to the countries who need it most.”
Malaria kills about 620,000 people each year, most of them young children.

It has been a massive, century-long, scientific undertaking to develop a vaccine that protects the body from the malaria parasite.
Trial data from preliminary studies in Burkina Faso showed the R21 vaccine was up to 80% effective when given as three initial doses, and a booster a year later.
But widespread use of the vaccine hinges on the results of a larger trial involving nearly 5,000 children, DW.
These had been expected to take place at the end of last year, but have still not been formally published. However, they have been shared with some government bodies in Africa, and scientists.
